
From the second century BC. The rings have been a show of love and fidelity of a couple. But what makes diamond one of the most precious stones in the world, that is thanks to its physical and chemical characteristics.
Chemical properties
 What is it made of?
What is it made of?
The diamond is composed of pure carbon atoms which under specific temperatures and pressures makes it the second allotrope of the most stable carbon after graphite, in short, it is crystallized coal.
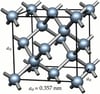 Forming
Forming
The creation of a diamond is through very specific conditions. It forms at 29,607,695 atmospheres of pressure and between 900 and 1300ºC. To arrive at such pressures and temperatures the diamonds are between 140 and 190 km below the terrestrial surface. This causes coal to combine in a cubic molecule which is a process that can last from 1 to 3.3 billion years as the diamond increases in size. Diamonds approach the surface by volcanic eruptions and changes in the Earth's mantle.
Physical properties
This transparent crystal of carbon atoms has some of the most fascinating physical characteristics that man has discovered.
Hardness
The diamond is the hardest material known to man, this is measured by the ease with which it is scratched, and it can only be carved by another diamond. The hardness of the diamond has been known since antiquity and it is the source of its name. The preservation of the diamond is due to this characteristic, because no matter the generations through which this gem passes it will remain intact if you have the right care.
Color
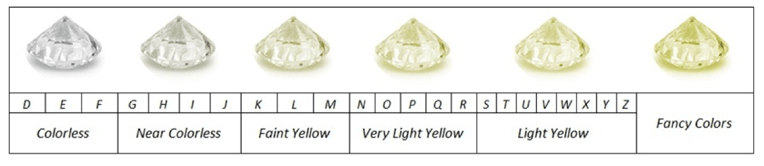
The ideal diamond covers the entire visible spectrum; that is to say, it transmits visible light but looks like a transparent crystal. The colors of a diamond are given by their defects and impurities, the most common is when the diamond has a yellow hue which is caused by the replacement of a carbon atom with one of nitrogen. GIA has established a scale from D to Z to identify the color of the diamond.
Optics
The brilliance of a diamond is the highest thanks to its hardness, is what makes it look alive. The reflections in the facets are perfect, because they are perfectly flat and its transparency which makes the light through it without losing its intensity. The fire or flashes of prismatic colors, present in transparent gems, are due to their dispersion which is the most important characteristic of the diamond, this causes the light to reflect and separate the colors from the spectrum.


History
The first time a proposal was made with this precious stone was in 1477, when Archduke Maximilian I of Hamburg proposed to the Duchess Maria of the house of Burgundy the diamond ring to showed his great love for her. This was the beginning of a tradition that has continued for more than 500 years promising true love.
 The best quality at the best price
The best quality at the best price